Abstract
Introduction: CD19-targeted chimeric antigen receptor T-cell therapy (CAR-T) has revolutionized the treatment of non-Hodgkin lymphomas (NHL). Due to underlying NHL, CAR-T therapy, and treatment of associated toxicities, CAR-T recipients are immunocompromised and, consequently, at high risk of infection. Evidence informing prophylaxis (PPx) is limited and often varies based on institutional practice. This study sought to determine the incidence of fungal infection after CAR-T in patients with NHL treated in Arizona and describe PPx strategies.
Methods: Using Mayo Clinic Arizona's (MCA), Banner MD Anderson's (BMDACC), and Banner University Medical Center's (BUMC) CAR-T databases, patients who received commercial CD19-targeted CAR-T for NHL between September 2018 and June 2022 were identified. Disease characteristics, prior and subsequent therapy, antimicrobial use, and infectious disease testing were collected. Early and late adverse events including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), hypogammaglobulinemia, and cytopenias were collected. The 2020 European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium consensus definitions were used to define probable and proven invasive fungal disease (IFD).
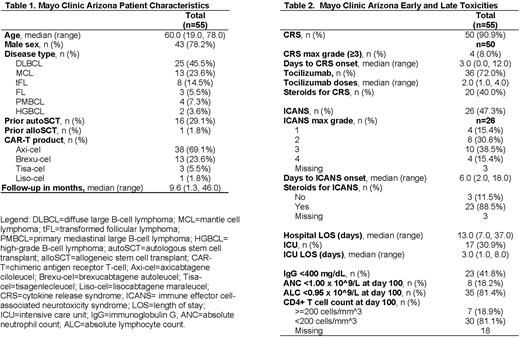
Results: A total of 55 patients from MCA met inclusion criteria (Table 1). The median age at infusion was 60 years (range 19-78) and median follow-up time was 9.6 months (range 1.3-46.0) post CAR-T infusion. Most patients were male (78.2%, n=43), had diffuse large B-cell lymphoma (45.5%, n=25), and received axicabtagene ciloleucel (69.1%, n=38). Early and late toxicities (Table 2) included grade ≥3 CRS (7.2%, n=4), grade ≥3 ICANS (25.5%, n=14), immunoglobulin G <400 mg/dL (41.8%, n=23) and a CD4 T-cell count <200 cells/mm^3 (81.1%, n=30). Thirty-six patients (65.4%) received tocilizumab, 29 patients (52.7%) received steroids for CRS and/or ICANS.
At MCA, two patients (3.6%) had mucocutaneous Candida infection, at day 10 and day 14 post-infusion, respectively. Four patients (7.3%) developed IFD, all of whom were in partial or complete response. Two (3.6%) had biopsy proven pulmonary IFD caused by Aspergillus and Coccidioides at day 64 and day 233 post-infusion, respectively. Two (3.6%) had probable pulmonary IFD caused by Aspergillus and Pneumocystis jirovecii at day 107 and day 209 post-infusion, respectively. These two patients died shortly after their IFD diagnosis, however also had proven COVID-19 infection and bacterial sepsis, respectively. Of those with IFD, only the patient with Pneumocystis jirovecii pneumonia (25%, n=1/4) had received steroids for the treatment of CAR-T related toxicity. No IFDs were identified by database review at Mayo Clinic in Rochester or Florida, thus leading our group to consider endemic risk factors in Arizona.
At BUMC, 3 patients (8.5%, n=3/35) developed pulmonary IFD, 2 with Aspergillus and 1 with Coccidioides. At BMDACC, 1 patient (3%, n=1/37) developed Saccharomyces of the tongue at day 30 while receiving fluconazole.
At MCA, antifungal PPx, most commonly with fluconazole, was used in those with neutropenia lasting >7 days (49%, n=27), whereas it was used routinely at BMDACC and BUMC for at least 180 days. All patients received trimethoprim-sulfamethoxazole PPx for at least 180 days unless an alternative was necessary. Patients at MCA, BUMC, and BMDACC are routinely screened for Coccidioides prior to CAR-T, although prior anti-B-cell therapy may impact these results. Serum Coccidioides antibody, complement fixation, and immunodiffusion were positive in only 1 MCA patient prior to receiving CAR-T and this patient had an uncomplicated course, although received extended PPx fluconazole.
Conclusions: Fungal infection after CAR-T for NHL appears to be uncommon. IFD tends to occur later, often beyond the typical time frame of antifungal PPx and/or caused by organisms not necessarily prevented by typical PPx regimens. Future studies are needed to individualize infectious disease screenings, monitoring, and antifungal PPx in this population.
Disclosures
Gaulin:DeciBio: Honoraria. Ulrickson:Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Vergidis:Cidara: Other: Principal Investigator on clinical trials; Scynexis: Other: Principal Investigator on clinical trials; Ansun: Other: Principal Investigator on clinical trials; Abbvie: Other: Data & Safety Monitoring Board. Wang:Novartis: Research Funding; Loxo@Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; MorphoSys: Research Funding; Genmab: Research Funding; Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees. Castro:Tmunity Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Kite Pharma: Research Funding. Palmer:Sierra Oncology: Consultancy; CTI BioPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Protagonist: Consultancy; PharmaEssentia: Consultancy, Research Funding. Lin:Gamida Cell: Consultancy; Bluebird Bio: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Novartis: Consultancy; Juno: Consultancy; Celgene: Consultancy, Research Funding; Merck: Research Funding; Takeda: Research Funding; Sorrento: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Vineti: Consultancy; Legend: Consultancy. Munoz:Pharmacyclics/Janssen: Speakers Bureau; Millennium: Research Funding; Portola: Research Funding; Physicians' Education Resource: Honoraria; Incyte: Research Funding; Celgene/Bristol Myers Squibb: Speakers Bureau; Kyowa Kirin: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy; Debiopharm: Consultancy; Epizyme: Consultancy; Fosun Kite: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Gilead: Research Funding; Genmab: Consultancy; Innovent: Consultancy; Janssen: Consultancy, Research Funding; Juno/Celgene: Consultancy; Karyopharm: Consultancy; MorphoSys/Incyte: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Pharmacyclics/AbbVie: Consultancy; Servier: Consultancy; Acrotech/Aurobindo: Speakers Bureau; AstraZeneca: Speakers Bureau; Genentech/Roche: Speakers Bureau; Seagen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Verastem: Speakers Bureau; Celgene: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Alexion: Consultancy; BeiGene: Consultancy, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Curio: Honoraria; Targeted Oncology: Honoraria; OncView: Honoraria; ADC Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal